Retatrutide
Retatrutide is not an approved medication and is therefore not legally available. Try a natural alternative to speed up your weight loss.
PhenQ supplement
Retatrutide: the Novel Solution for Weight Loss
American pharmaceutical giant Eli Lilly and Company is continuing its work on developing increasingly effective treatments for diabetes and obesity. Following the market success of their Mounjaro injections, there has been a wave of online discussion about the medicine retatrutide, which has already passed several successful trials and is soon expected to receive initial approvals from national regulators in the US, UK, EU and Australia.
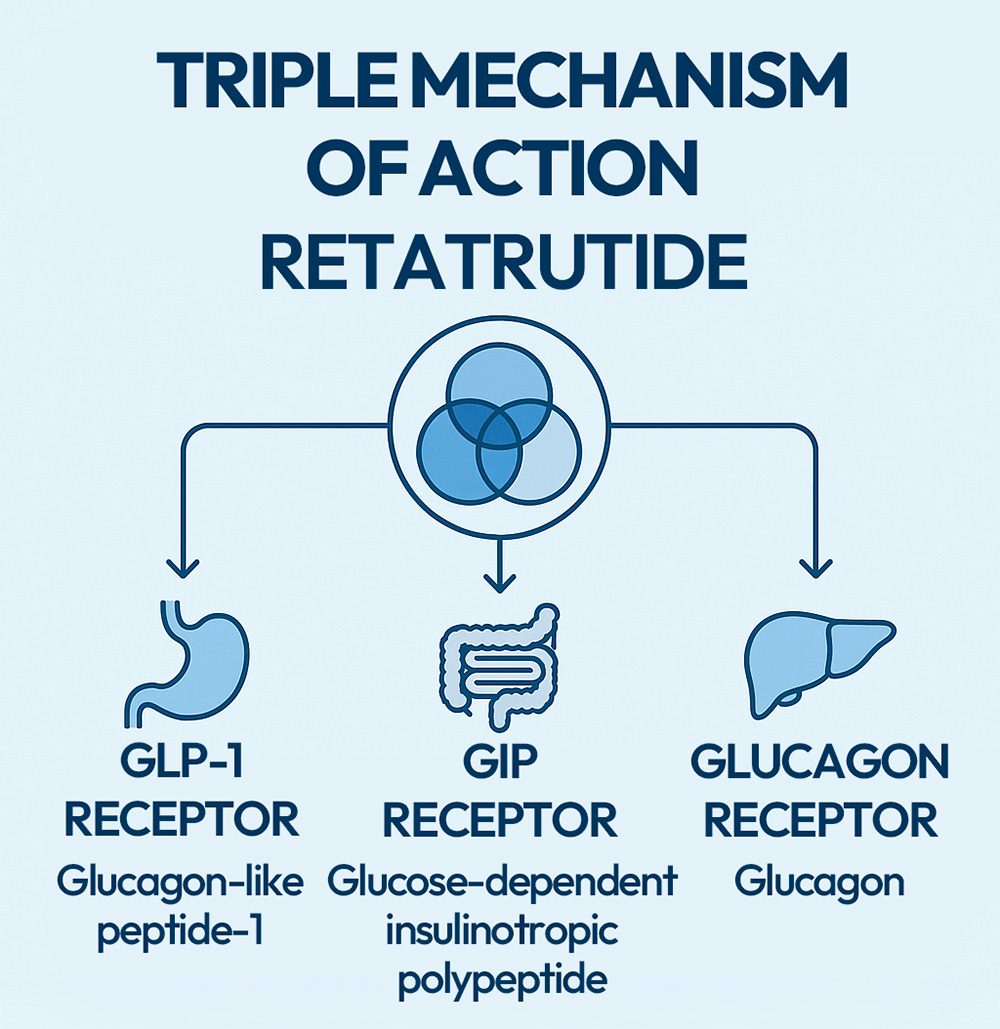
Retatrutide is a unique medicine because it is the first triple agonist of the GLP-1, GIP and glucagon receptors.[1] It has been developed and is being tested as a treatment that can lower blood sugar levels with minimal risk of hypoglycaemia, as well as promote significant weight loss in people with obesity.
How Does Retatrutide Work?
Its action mechanism is characterised by three key aspects:
- Unique three-dimensional action. Retatrutide is a triple hormone receptor agonist. It activates the receptors for three key hormones: GLP-1, GIP and glucagon. These hormones play a vital role in how your body manages blood sugar, appetite and metabolism. By stimulating all three pathways simultaneously, it provides a significant metabolic boost.
- Decreased appetite and cravings. It reduces appetite by acting on the brain’s hunger centres, making you feel fuller for longer. In addition, it delays gastric emptying This leads to reduced calorie intake, which is a key factor in the significant weight loss observed in trials. Put simply, you won’t want to eat too much. Also, you may naturally feel disgusted by high-fat and high-sugar foods.
- Increased calorie burn. Glucagon activation increases energy expenditure, meaning your body burns more calories, even at rest (known as basal metabolism). When combined with reduced food intake, this promotes ongoing fat loss and improves metabolic health. Reta is currently the only anti-obesity experimental medication that activates glucagon receptors.
The safety profile of Reta has been reported to be similar to other incretin-based therapies.[2] In this context, it should be noted that a significant concern has recently emerged regarding the chronic activation of glucagon receptors, one of the actions of retatrutide, which may reduce protein synthesis in muscles. However, this does not apply to retatrutide. New studies have shown the following:
“a greater proportion of lean mass is not lost with retatrutide despite the overall increased weight loss”[3]
Retatrutide as a Synthetic Peptide
Retatrutide is a synthetic analogue of the human peptide hormones GLP-1, GIP, and glucagon. This substance is most similar to GIP, which is responsible for stimulating insulin production and also acts as a fat absorption inhibitor. Thus, this substance primarily affects GIP receptors.
When it enters the body, the synthetic peptide acts in the same way as the natural one. However, natural peptides have a very short half-life (2-3 minutes for GLP-1; 3-6 minutes for glucagon; 15-16 minutes for GIP), while retatrutide is able to affect receptors for about 6 days.
How to Get Retatrutide?
When searching online or asking ChatGPT, you might come across some sites claiming to sell Retatrutide as a novel peptide. Be cautious — buying from these sources could have serious, unintended consequences. It’s clear that any medication still in the clinical trial phase and not approved anywhere globally cannot be legally sold.
Furthermore, it is highly likely that illegal sellers are passing off inulin or other anti-diabetic substances as retatrutide. The only legal way to use retatrutide as of 2025, is to become a participant of the relevant clinical trial. There are very few such participants (up to several thousand) compared to the number of patients with type 2 diabetes or obesity. If you cannot wait for the new medicine to become available, discuss with your doctor the purchase of a suitable alternative (medicine or dietary supplement).
Is It Possible to Buy Retatrutide in Australia?
At present, Retatrutide is not available for purchase in Australia. You will be unable to find it in local pharmacies like Chemist Warehouse. However, patients who are overweight still have plenty of options for weight loss. If you’re seeking a trustworthy weight-loss solution with minimal to no side effects, PhenQ might be worth considering.
Availability of Retatrutide in Australia: Future Prospects
The medication will not be available for sale until 2026 (most likely in early 2027). It will be sold in Australia with a prescription from a doctor who will monitor the indications for its use. For this reason, it is highly unlikely that retatrutide will be available for purchase online even after approval.
Price of Retatrutide
We will not comment on the prices of dubious powders and injections that allegedly contain retatrutide. The price of such “medicines” is set without any logic. If we were to make any predictions about the cost of retatrutide after its approval, it would be best to use the current price of Mounjaro as a basis — 350-450 AUD per syringe pen. Retatrutide may cost 10-15% more, which would allow for a clear price distinction between different generations of drugs.
Dosage of Retatrutide
As of 2025, it is known about five dosages of Reta, namely:
- 1 mg;
- 2 mg;
- 4 mg;
- 8 mg;
- 12 mg.
The initial dose is 1 or 2 mg. The dosage is increased every four weeks.
How to Take It?
This is expected to be a once-weekly subcutaneous injection. Like existing medications such as Saxenda, Ozempic, Mounjaro and Wegovy, it will be available as a pen-style injector. The injection can be administered in your thigh, upper arm or stomach area.
Side Effects of Retatrutide
Side effects of the substance mostly affect the gastrointestinal tract. As a rule, they are mild to moderate and should go away or ease on their own. However, severe adverse events are also possible.
The unique side effect of this medication when compared to similar meds like semaglutide and dulaglutide, is its ability to increase heart rate.
Retatrutide vs Ozempic (semaglutide)
| Criteria | Retatrutide | Ozempic (Semaglutide) |
|---|---|---|
| Active substance | Retatrutide (GLP-1/GIP/glucagon agonist) | Semaglutide (GLP-1 agonist) |
| Approval | Not approved (as of 2025) | Approved (TGA, FDA, EMA, etc.) |
| Manufacturer | Eli Lilly | Novo Nordisk |
| Indications | Under trial for obesity & type 2 diabetes | Type 2 diabetes (also used off-label for weight loss) |
| Possible effects | Greater weight loss, improved metabolism | Blood sugar control, moderate weight loss |
| Side effects | Nausea, vomiting, GI upset, heart rate increase (trial phase) | Nausea, vomiting, diarrhoea, constipation |
Retatrutide vs Mounjaro (tirzepatide)
| Feature | Retatrutide (Triple‑Agonist) | Tirzepatide (Dual‑Agonist) |
| Mechanism of action | Agonist at GIP, GLP‑1, and glucagon receptors | Agonist at GIP and GLP‑1 receptors |
| Weight loss results | –24.2 % with 12 mg (48 wk); plateau not reached [1][2], this means that full potential had not yet been shown | Up to –20.9 % with 15 mg at week 72 [5]. Other study showed a 26% weight reduction in the course of the 84-week program[6] |
| Cardiometabolic effects | Lowers blood pressure, glucose, lipids; 72 % of pre-diabetics reverted to normoglycemia [1] | Significant HbA1c reduction; improved cardiometabolic outcomes [5] |
| Safety profile | Gastrointestinal side effects mild/moderate; heart rate increase is possible; dose‑escalation recommended. | Similar gastrointestinal effects; standard safety profile |
| Phase 3 trials | Ongoing TRIUMPH‑1–5 targeting obesity, type 2 diabetes, cardiovascular disease, osteoarthritis, obstructive sleep apnea etc. [4] | Already approved for weight management under Zepbound, Mounjaro is also approved for weight loss in many countries |
FAQ
First and foremost, you should meet the eligibility criteria (BMI 30 or higher or 27 or higher with overweight-related health conditions). Then you can browse the specialised Lilly’s website (trials.lilly.com) to find the necessary trial. However, we haven’t found a way to do this for Australian residents.
No. Legally this is not possible.
It is unknown at the moment. Currently some websites advertise retatrutide-containing medication called Vigelan. It is manufactured by a suspicious company named SunSci. We’ve visited their website and revealed that they have no registered address, and they also offer another allegedly illegal product with tirzepatide called Twincret.
Visiting Reddit may give the impression that Retatrutide (or simply Reta, as users of this forum refer to it) has been approved for a long time. There you will find numerous posts about the positive effects of this substance and even before and after photos. The problem is that 90% of the threads dedicated to this new development are commercial and ultimately boil down to offers to buy a cheap Chinese version of retatrutide. It is obvious that in this context, it is impossible to trust positive reviews.
No, this is not a steroid. This is a peptide substance composed of amino acids.
The price of retatrutide will ultimately depend on:
- The cost of competing meds (primarily Wegovy and Ozempic)
- Consumer price levels
- The company’s financial position
- Marketing research results
The cost of a month’s treatment for a patient will depend on:
- Maintenance dose (similar drugs have a wide price range between dosages, the bigger the dose the higher the price)
- Available packaging (packaging with a large number of pens is always more cost-effective, however, it is not known whether multiple-pen packs with Reta will be offered)
- Tolerability of the therapy (individual reactions to the medication may require additional costs, for example, for OTC nausea treatments)
According to the research data, significant weight loss is achieved after around 45 weeks of taking retatrutide. This is much faster than the results achieved with current obesity drug therapies. Clearly, the first results will be visible much earlier.
Specific information on the storage conditions and shelf life of retatrutide will be published when the drug is submitted for approval to the FDA and EMA. These are likely to be similar to the storage conditions for tirzepatide, given that the two drugs are very similar. If so, the injection solution will need to be stored in a refrigerator to prevent freezing, or at room temperature for no longer than 30 days.
No, the dosage should be elevated gradually, and the scheme of use should be more or less identical for all patients. Proper dosing is paramount, starting from moderate or maximal amounts will lead to side effects of increased severity.
No (lifestyle and diet modifications are still necessary), but it should be much more effective than its competitors. Scientists have noted that “although the drug is currently in the testing phase it seems to be the answer for future obesity epidemic.”
Sources:
- Phase 2 Results of Retatrutide in the New England Journal of Medicine
- Lilly’s Press Release on Phase 2 Retatrutide Results
- ScienceDirect: Dual/Triple Agonist Therapies for Obesity
- ClinicalTrials.gov: Study Record NCT05929066 for Retatrutide
- MDPI: Recent Advances in Multi-Agonist Obesity Therapies
- Medical News Today: Mounjaro and Weight Loss Insights
- ResearchGate: Retatrutide Literature Review